Your Is boiling water endothermic or exothermic images are ready in this website. Is boiling water endothermic or exothermic are a topic that is being searched for and liked by netizens now. You can Download the Is boiling water endothermic or exothermic files here. Download all royalty-free photos.
If you’re searching for is boiling water endothermic or exothermic images information linked to the is boiling water endothermic or exothermic interest, you have come to the right site. Our website always gives you suggestions for seeking the maximum quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
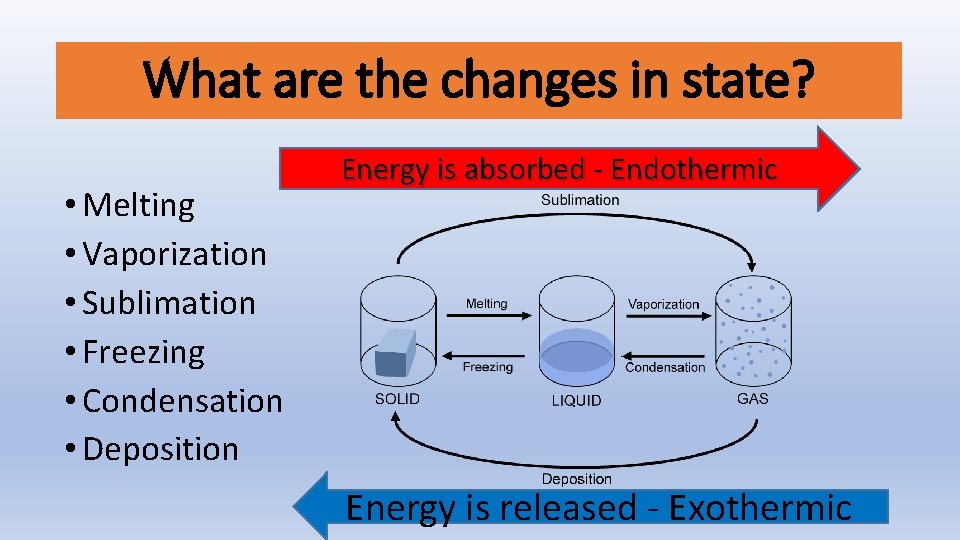
Is Boiling Water Endothermic Or Exothermic. We can all appreciate that water does not spontaneously boil at room temperature. Which of the following processes are exothermic. Taken away from combustion of the fuel to heat up the water. We can all appreciate that water does not spontaneously boil at room temperature.
 Day 1 Thermo Warm Up 8 1 Determine If The Following Is Chemical Or Physical 1 Boiling Water 2 Rusting 3 Evaporation 4 Decomposing 5 Baking 6 Freezing Ppt Download From slideplayer.com
Day 1 Thermo Warm Up 8 1 Determine If The Following Is Chemical Or Physical 1 Boiling Water 2 Rusting 3 Evaporation 4 Decomposing 5 Baking 6 Freezing Ppt Download From slideplayer.com
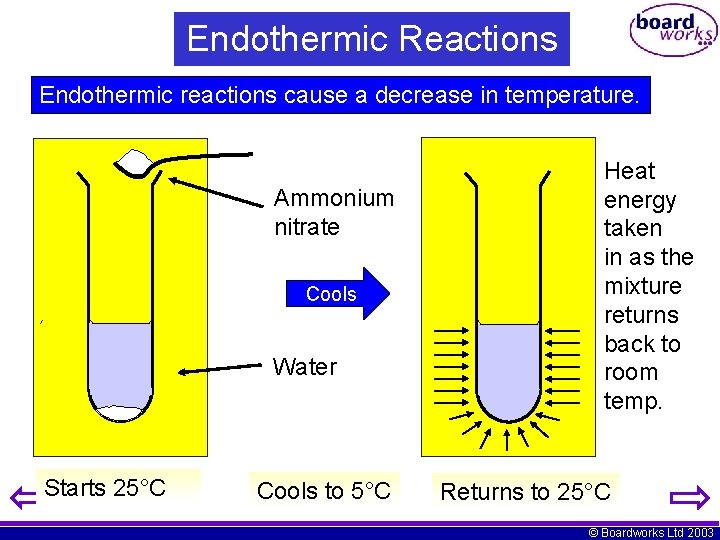
Instead we must heat it. Boiling water is exothermic. It is less intuitive to grasp that when a gas condenses to a liquid heat is given off and the process is exothermic. Examples of endothermic reactions boiling water making popcorn in a microwave oven. Vaporization like melting requires energy to be put in for the molecules to change state from liquid to gas. For The Condensation Of Steam.
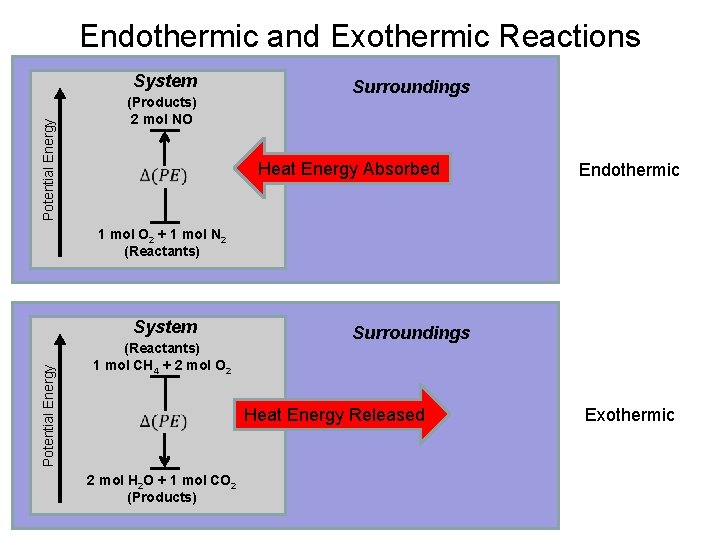
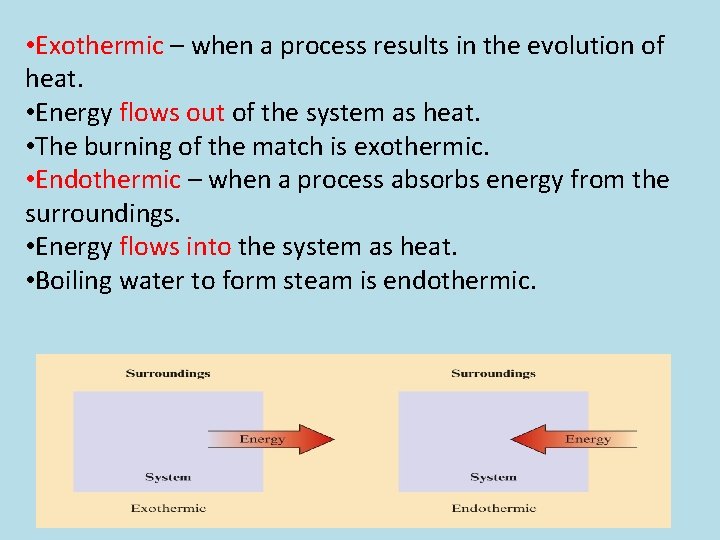
These are known as exothermic.
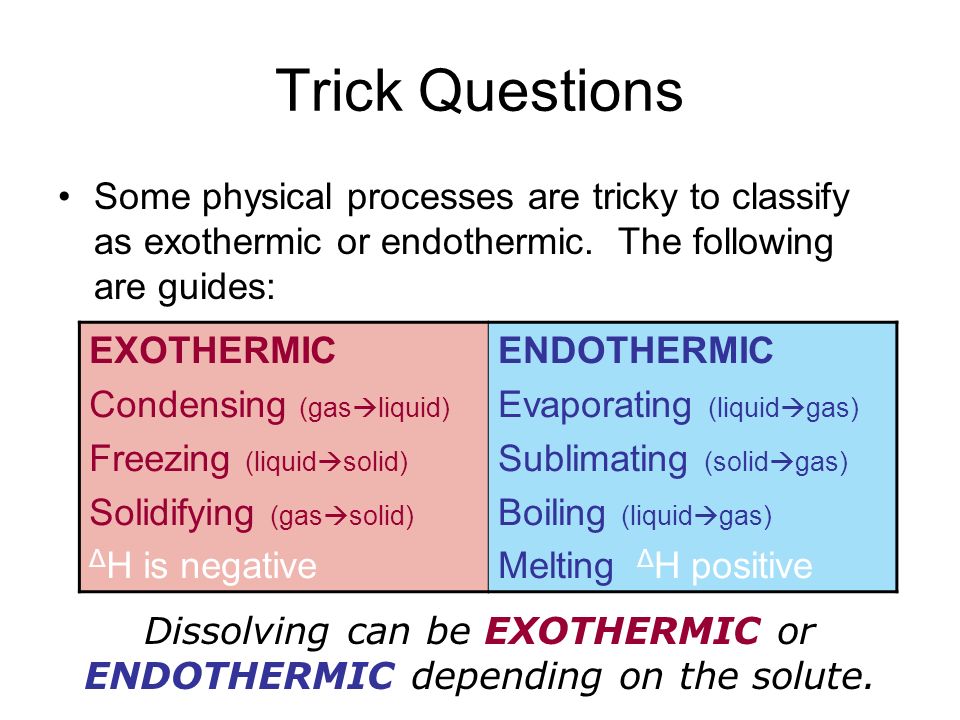
If a substance eg. Because we must add heat boiling water is a process that chemists call endothermic. These are known as exothermic. Which of the following processes are exothermic. Instead we must heat it. Stay tuned to BYJUS to learn similar questions and important points related to the endothermic process.
 Source: slideplayer.com
Source: slideplayer.com
Because we must add heat boiling water is a process that chemists call endothermic. On the other hand if boiling is a verb - is the action of taking water at ambient temperature and bringing it to the boil endothermic or exothermic. It simply means that 347kJ of energy has been used ie. Boiling water is exothermic. This problem has been solved.
 Source: brainstudy.info
Source: brainstudy.info
These are known as exothermic. Explore more NEET questions here. This is the very definition of an endothermic process. This problem has been solved. So that makes it endothermic.
 Source: chemistrylearner.com
Source: chemistrylearner.com
This is the very definition of an endothermic process. It simply means that 347kJ of energy has been used ie. Is The Boiling Of Water Endothermic Or Exothermic. Therefore this must be exothermic. The water on your skin takes heat from your body to evaporate.
 Source: brainstudy.info
Source: brainstudy.info
For The Condensation Of Steam. Vaporization like melting requires energy to be put in for the molecules to change state from liquid to gas. On the other hand if boiling is a verb - is the action of taking water at ambient temperature and bringing it to the boil endothermic or exothermic. Our experience makes it easy for us to realize that to boil water or any liquid and thereby convert into a gas heat is required and the process is endothermic. Exothermic state changes liquid to solid freezing.
 Source: brainstudy.info
Source: brainstudy.info
Examples of exothermic reactions a burning match the reaction inside a chemical heat pack burning rocket fuel combustion reaction. Because we must add heat boiling water is a process that chemists call endothermic. Vaporization like melting requires energy to be put in for the molecules to change state from liquid to gas. This is the very definition of an endothermic process. Clearly if some processes require heat others must give off heat when they take place.
 Source: slideplayer.com
Source: slideplayer.com
On the other hand if boiling is a verb - is the action of taking water at ambient temperature and bringing it to the boil endothermic or exothermic. How can you tell. That makes them endothermic. You must add heat to boil soup and evaporate water. As said above water heating up would be endothermic.
 Source: slidetodoc.com
Source: slidetodoc.com
This problem has been solved. Clearly if some processes require heat others must give off heat when they take place. This problem has been solved. You can feel it giving out heat as you approach the container it is in - say a kettle. It is less intuitive to grasp that when a gas condenses to a liquid heat is given off and the process is exothermic.
 Source: slidetodoc.com
Source: slidetodoc.com
How can you tell. You can determine this by heating liquid water on the stove it will boil into a gas. Endothermic means heat in. We can all appreciate that water does not spontaneously boil at room temperature. Explore more NEET questions here.
Source: encrypted-tbn0.gstatic.com
That makes them endothermic. It simply means that 347kJ of energy has been used ie. Jul 18 2010. Thus liquid water needs to receive energy from an outside source to evaporate. The boiling of water is an endothermic process.
 Source: slideplayer.com
Source: slideplayer.com
These are known as exothermic. We can all appreciate that water does not spontaneously boil at room temperature. So that makes it endothermic. Stay tuned to BYJUS to learn similar questions and important points related to the endothermic process. The water on your skin takes heat from your body to evaporate.
Source: quora.com
Jul 18 2010. So that makes it endothermic. We can all appreciate that water does not spontaneously boil at room temperature. Clearly if some processes require heat others must give off heat when they take place. Instead we must heat it.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Then - how do you do this. Which of the following processes are exothermic. Thus liquid water needs to receive energy from an outside source to evaporate. Likewise when liquid water freezes heat is given off. Boiling water is exothermic.
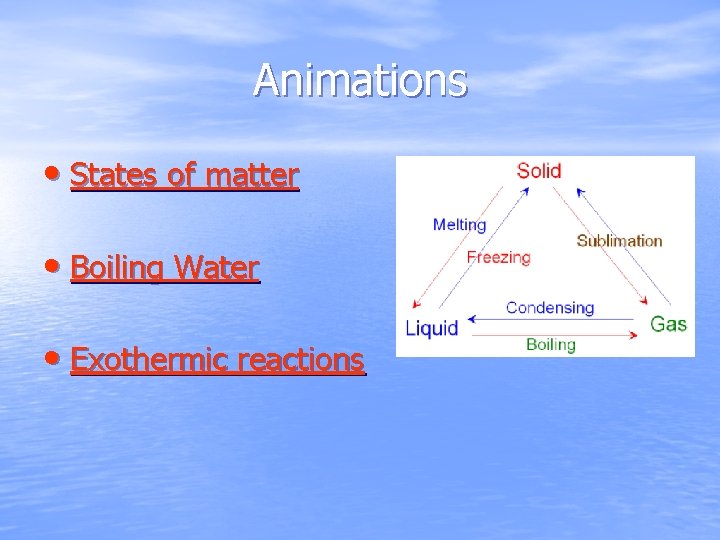 Source: slidetodoc.com
Source: slidetodoc.com
For The Condensation Of Steam. The water on your skin takes heat from your body to evaporate. The temporary bonds between liquid molecules must be broken for the liquid to change to a gas this requires energy to be put into the system endothermic Exothermic means heat out. An endothermic process is defined as the chemical reaction in which heat energy is absorbed from its surrounding in the form of heat. If playback doesnt begin shortly try restarting your device.
 Source: learn.lif.co.id
Source: learn.lif.co.id
Thus liquid water needs to receive energy from an outside source to evaporate. Because we must add heat boiling water is a process that chemists call endothermic. Instead we must heat it. Water changes between two different phases but without undergoing chemical decomposition then this is termed a phase transition. You must add heat to boil soup and evaporate water.
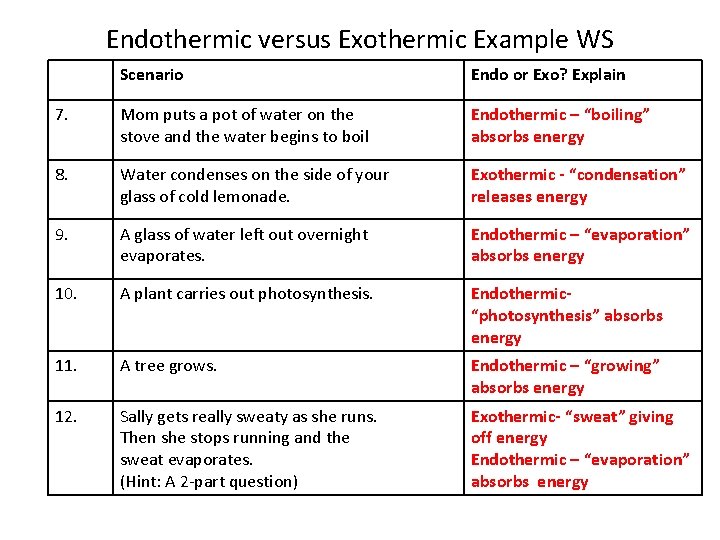 Source: brainstudy.info
Source: brainstudy.info
Show transcribed image text. Clearly if some processes require heat others must give. The temporary bonds between liquid molecules must be broken for the liquid to change to a gas this requires energy to be put into the system endothermic Exothermic means heat out. Every phase transition is an endothermic or. We can all appreciate that water does not spontaneously boil at room temperature.
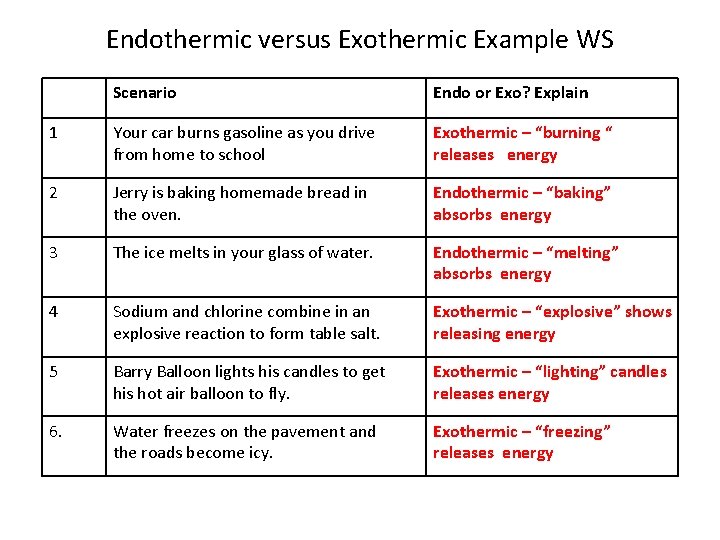 Source: brainstudy.info
Source: brainstudy.info
If a substance eg. Instead we must heat it. Exothermic state changes liquid to solid freezing. This is the very definition of an endothermic process. This problem has been solved.
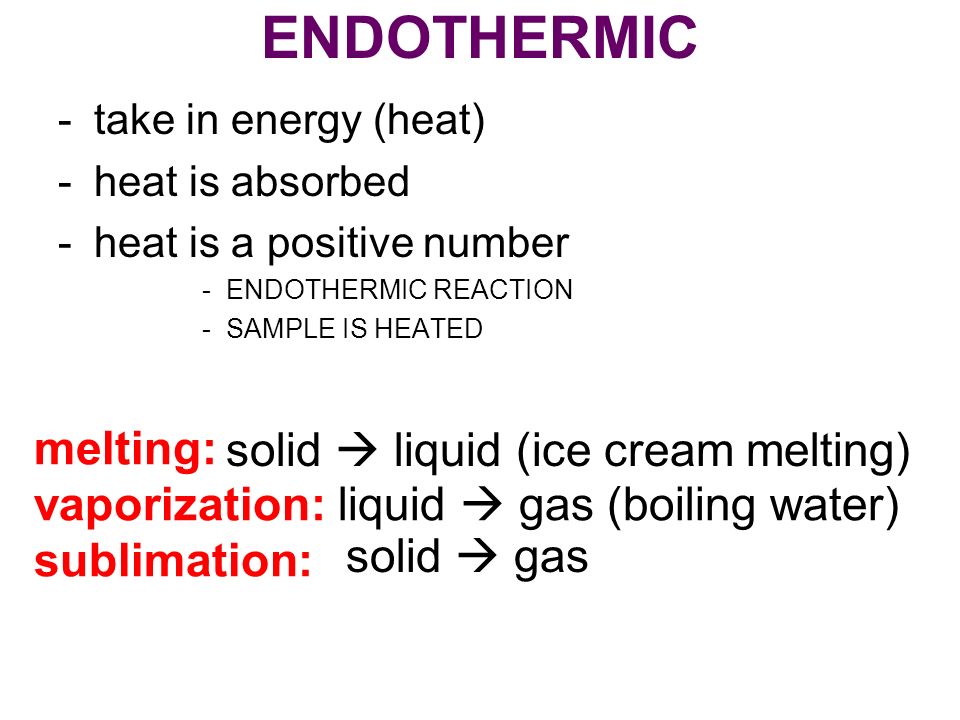 Source: slideplayer.com
Source: slideplayer.com
An endothermic process is defined as the chemical reaction in which heat energy is absorbed from its surrounding in the form of heat. You must add heat to boil soup and evaporate water. It simply means that 347kJ of energy has been used ie. Is The Boiling Of Water Endothermic Or Exothermic. Show transcribed image text.
 Source: brainstudy.info
Source: brainstudy.info
Taken away from combustion of the fuel to heat up the water. Clearly if some processes require heat others must give. If playback doesnt begin shortly try restarting your device. Endothermic means heat in. Explore more NEET questions here.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is boiling water endothermic or exothermic by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






