Your Is evaporating alcohol endothermic or exothermic images are ready. Is evaporating alcohol endothermic or exothermic are a topic that is being searched for and liked by netizens now. You can Download the Is evaporating alcohol endothermic or exothermic files here. Download all free vectors.
If you’re searching for is evaporating alcohol endothermic or exothermic pictures information linked to the is evaporating alcohol endothermic or exothermic interest, you have visit the ideal site. Our site always provides you with hints for seeing the highest quality video and picture content, please kindly surf and locate more enlightening video content and graphics that match your interests.
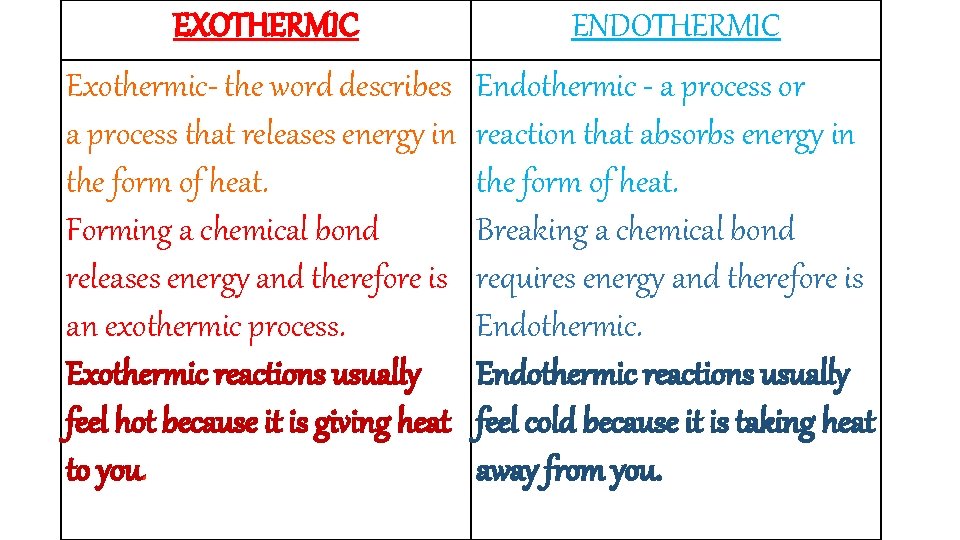
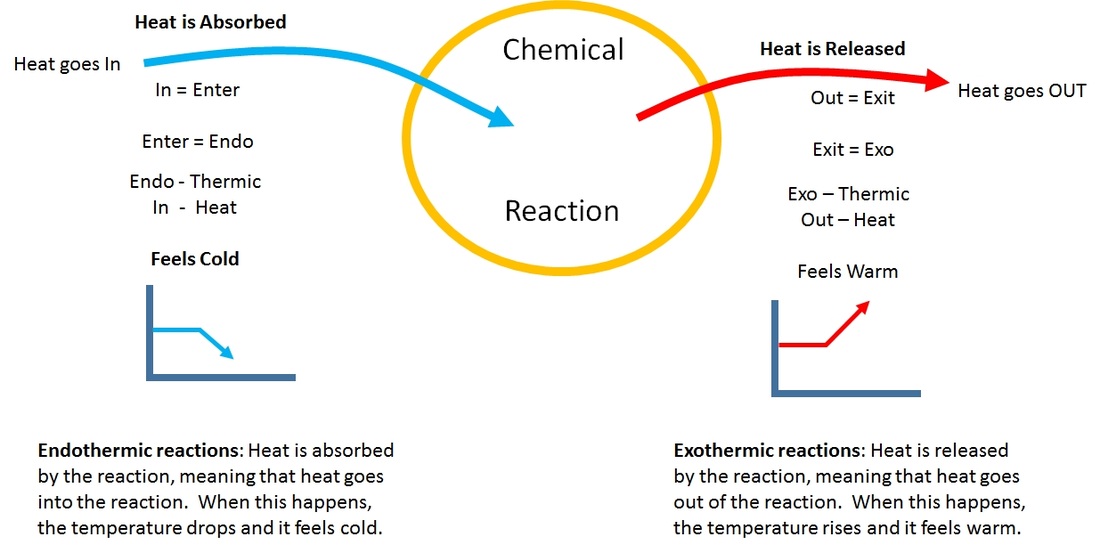
Is Evaporating Alcohol Endothermic Or Exothermic. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. Is isopropyl alcohol evaporating from skin process exothermic or endothermic. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure. 1 A system where matter cannot be added to products and reactants.
 Solved Is Each Process Exothermic Or Endothermic From numerade.com
Solved Is Each Process Exothermic Or Endothermic From numerade.com
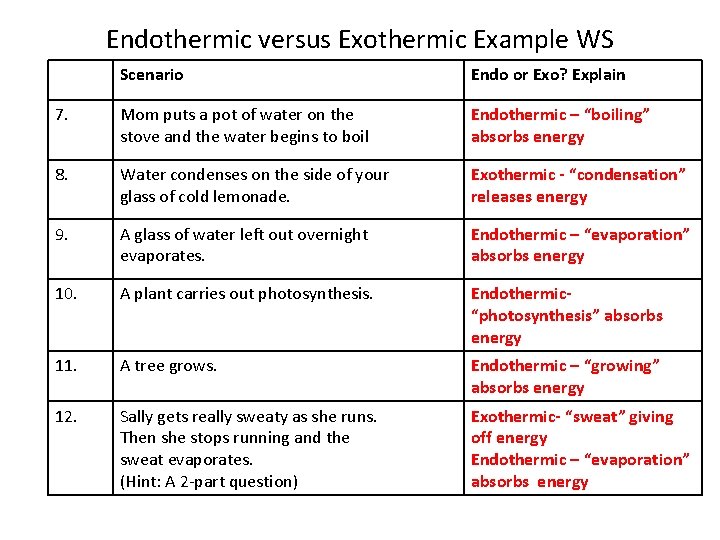
These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. Defrosting frozen food d. If you put rubbing alcohol onto your skin it will evaporate and your skin will feel cold. Natural gas burning on a stove. Which process is exothermic. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy.
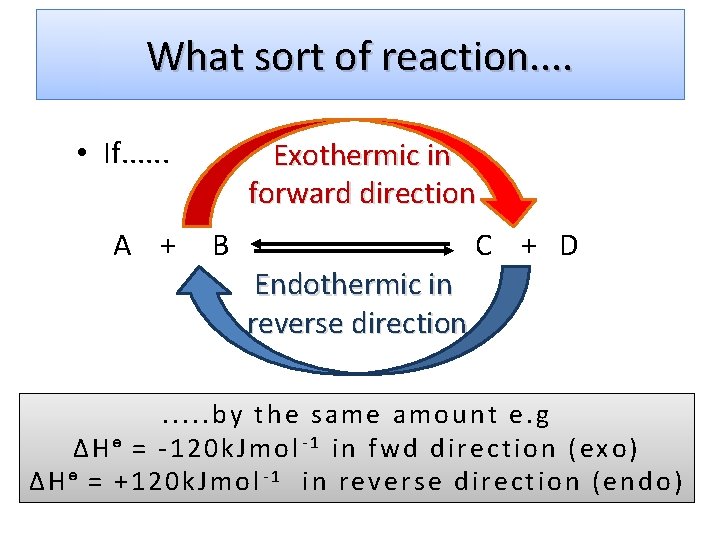
I would thus guess that formation of most carboxylic esters from carboxylic acids and alcohols would be slightly exothermic or slightly endothermic depending on structure.
These interactions must be broken to vaporize the. Is Melting exothermic or endothermic. In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings. Natural gas burning on a stove. Calculate the work associated with this process. How many years does it take to become a banker.

These interactions must be broken to vaporize the. If you put rubbing alcohol onto your skin it will evaporate and your skin will feel cold. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. What is the frequency of a helium-neon laser with a wavelength of 6473 nm. A 14 L sample of Ne gas contracts to 3 L due to external pressure of 15 atm.
 Source: slidetodoc.com
Source: slidetodoc.com
Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy. Freezing rain drops b. These interactions must be broken to vaporize the. When the water and methanol are mixed together some of the existing hydrogen bonding water-water or methanol-methanol is disturbed and now there is hydrogen bonding between water and methanol. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy.
 Source: slidetodoc.com
Source: slidetodoc.com
Isopropyl alcohol evaporating from skin. Endothermic reactions end up with an overall positive heat of reaction. When the methanol and water are separate they both exhibit hydrogen bonding with themselves. Subliming dry ice 19. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy.

Freezing rain drops b. Is the evaporation of alcohol endothermic or exothermic. Isopropyl alcohol evaporating from skin. In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings. Indicate the sign of H.
 Source: pinterest.com
Source: pinterest.com
If you put rubbing alcohol onto your skin it will evaporate and your skin will feel cold. Isopropyl alcohol evaporating from skin. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____. Is the evaporation of alcohol endothermic or exothermic. Endothermic reactions end up with an overall positive heat of reaction.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
Natural gas burning on a stove. Is the process of isopropyl alcohol evaporating from skin endothermic or exothermic. Is isopropyl alcohol evaporating from skin process exothermic or endothermic. Is the chemical reaction in a hot pack often used to treat sore muscles endothermic or exothermic. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure.
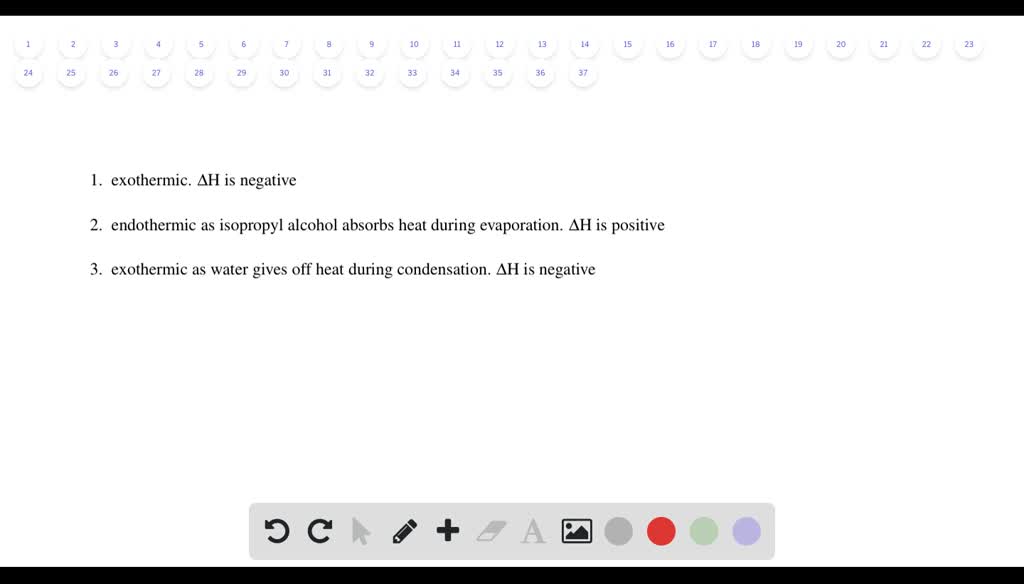 Source: numerade.com
Source: numerade.com
How many electrons will be in the correctly drawn Lewis Structure for CO. How many years does it take to become a banker. Is Melting exothermic or endothermic. What is the frequency of a helium-neon laser with a wavelength of 6473 nm. Which process is exothermic.
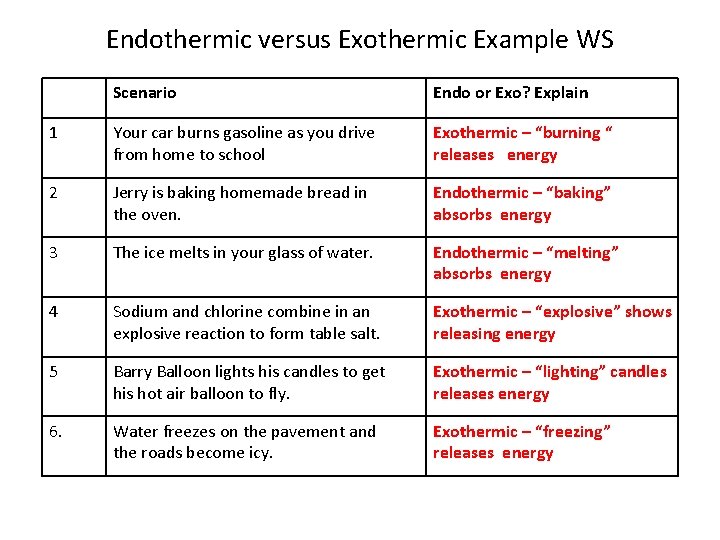 Source: slidetodoc.com
Source: slidetodoc.com
Is isopropyl alcohol evaporating from skin process exothermic or endothermic. To define endothermic and evaporation is endothermic we need something like this. A 14 L sample of Ne gas contracts to 3 L due to external pressure of 15 atm. In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____.
 Source: slideplayer.com
Source: slideplayer.com
Is isopropyl alcohol evaporating from skin process exothermic or endothermic. Is each process exothermic or endothermic. How many years does it take to become a banker. Endothermic reactions end up with an overall positive heat of reaction. Is the evaporation of alcohol endothermic or exothermic.
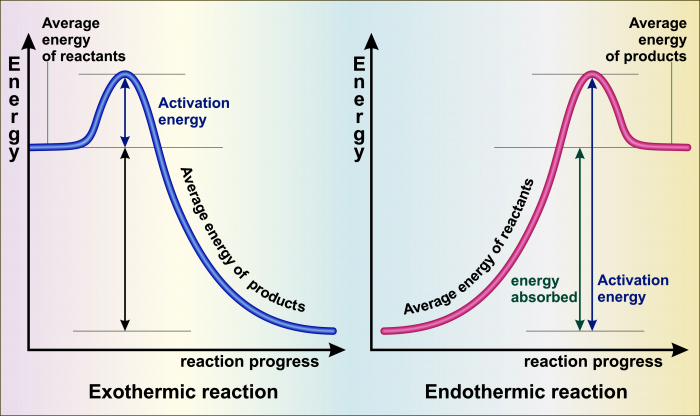 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
A 14 L sample of Ne gas contracts to 3 L due to external pressure of 15 atm. Endothermic reactions end up with an overall positive heat of reaction. Is Melting exothermic or endothermic. 1 A system where matter cannot be added to products and reactants. Is the freezing of water endothermic or exothermic.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Which process is exothermic. How many electrons will be in the correctly drawn Lewis Structure for CO. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure. Is the process of isopropyl alcohol evaporating from skin endothermic or exothermic. Freezing rain drops b.

It is endothermic since it takes in heat from its surroundings to go from a liquid to a gas. Is the evaporation of alcohol endothermic or exothermic. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____. When the methanol and water are separate they both exhibit hydrogen bonding with themselves. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
Indicate the sign of u001fH. Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy. Defrosting frozen food d. Subliming dry ice 19. These interactions must be broken to vaporize the.
 Source: slidetodoc.com
Source: slidetodoc.com
Isopropyl alcohol evaporating from skin. Endothermic reactions end up with an overall positive heat of reaction. Is the chemical reaction in a hot pack often used to treat sore muscles endothermic or exothermic. Is the freezing of water endothermic or exothermic. When the water and methanol are mixed together some of the existing hydrogen bonding water-water or methanol-methanol is disturbed and now there is hydrogen bonding between water and methanol.
 Source: slidetodoc.com
Source: slidetodoc.com
Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Endothermic reactions end up with an overall positive heat of reaction. Is the process of isopropyl alcohol evaporating from skin endothermic or exothermic. Evaporation is an endothermic process. Subliming dry ice 19.
 Source: slidetodoc.com
Source: slidetodoc.com
These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____. To define endothermic and evaporation is endothermic we need something like this. When the methanol and water are separate they both exhibit hydrogen bonding with themselves. Is the evaporation of alcohol endothermic or exothermic.
 Source: numerade.com
Source: numerade.com
Evaporation is an endothermic process. Problem 51 Medium Difficulty. Indicate the sign of H. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure.

Indicate the sign of H. How many years does it take to become a banker. It is endothermic since it takes in heat from its surroundings to go from a liquid to a gas. Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy. Evaporation is an endothermic process.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is evaporating alcohol endothermic or exothermic by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





