Your Is evaporation of ethanol endothermic or exothermic images are ready. Is evaporation of ethanol endothermic or exothermic are a topic that is being searched for and liked by netizens now. You can Find and Download the Is evaporation of ethanol endothermic or exothermic files here. Download all royalty-free vectors.
If you’re looking for is evaporation of ethanol endothermic or exothermic pictures information related to the is evaporation of ethanol endothermic or exothermic topic, you have pay a visit to the right blog. Our site frequently provides you with hints for downloading the highest quality video and picture content, please kindly search and locate more enlightening video articles and images that fit your interests.
Is Evaporation Of Ethanol Endothermic Or Exothermic. In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings. There must be heat added or absorbed from the environment to cook the egg or any other food item. The reasons esterifications are often heated are 1 to increase the rate of reaction and 2 to distill off water as it forms. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy.
 Exothermic Endothermic Reactions Poster Chemistry Lessons Teaching Chemistry Chemistry Classroom From pinterest.com
Exothermic Endothermic Reactions Poster Chemistry Lessons Teaching Chemistry Chemistry Classroom From pinterest.com
2 A system that can exchange heat with the surroundings. How many years does it take to become a banker. There must be heat added or absorbed from the environment to cook the egg or any other food item. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure. To define endothermic and evaporation is endothermic we need something like this. Endothermic heat absorbed or in something exothermic heat being released or sent out.
Endothermic heat absorbed or in something exothermic heat being released or sent out.
The particles that make up a liquid sample will have less. Is the evaporation of alcohol endothermic or exothermic. Sweat aqheat-sweat g Endothermic means that you need to put heat energy in to make the reaction happen. The reasons esterifications are often heated are 1 to increase the rate of reaction and 2 to distill off water as it forms. Feb 9 2012. Intermolecular bonds in the liquid in order to allow molecules to escape into the gas phase.
 Source: slideplayer.com
Source: slideplayer.com
Sweat aqheat-sweat g Endothermic means that you need to put heat energy in to make the reaction happen. Vaporization of liquid alcohol or any liquid is an endothermic process. C₃H₈O 52 kJ C₃H₈O g. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. As the reaction says that the energy is added to the system so it is endothermic.
Source: quora.com
Is a hot cup of coffee system cools on a countertop endothermic or exothermic. Some water-water H-bonds and ethanol-ethanol H-bonds are broken and some water-ethanol hydrogen bonds are formed. Is Melting exothermic or endothermic. The result is less heat when the reaction is done. We review their content and use your feedback to keep the quality high.
 Source: pinterest.com
Source: pinterest.com
View the full answer. Endothermic heat absorbed or in something exothermic heat being released or sent out. Is Melting exothermic or endothermic. Is the chemical reaction in a hot pack often used to treat sore muscles endothermic or exothermic. 2 A system that can exchange heat with the surroundings.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
Because molecules are on the inside of. Evaporation is an endothermic process and thus requires energy. Is a hot cup of coffee system cools on a countertop endothermic or exothermic. Sweat aqheat-sweat g Endothermic means that you need to put heat energy in to make the reaction happen. In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings.
Source: wordwall.net
Is the chemical reaction in a hot pack often used to treat sore muscles endothermic or exothermic. An endothermic change is a change that takes in heat. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Feb 9 2012. In addition to the formation of new compounds the chemical reaction that takes place when ethanol is burnt also releases a lot of heat 2777 kJ per mole of ethanol.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
C₃H₈O 52 kJ C₃H₈O g. Endothermic reactions end up with an overall positive heat of reaction. 2 A system that can exchange heat with the surroundings. Since Energy is Absorbed to break the bonds before it can become a gas that makes Evaporation an Endothermic Process. Endothermic heat absorbed or in something exothermic heat being released or sent out.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Since Energy is Absorbed to break the bonds before it can become a gas that makes Evaporation an Endothermic Process. 435 1337 Views. Condensation would be exothermic. So for an endothermic reaction it would absorb the heat of the surrounding to provide energy for said reaction. As the reaction says that the energy is added to the system so it is endothermic.
 Source: pinterest.com
Source: pinterest.com
How many years does it take to become a banker. View the full answer. Is the freezing of water endothermic or exothermic. The reasons esterifications are often heated are 1 to increase the rate of reaction and 2 to distill off water as it forms. There must be heat added or absorbed from the environment to cook the egg or any other food item.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Evaporation is an endothermic process and thus requires energy. There must be heat added or absorbed from the environment to cook the egg or any other food item. Endothermic heat absorbed or in something exothermic heat being released or sent out. Since Energy is Absorbed to break the bonds before it can become a gas that makes Evaporation an Endothermic Process. So for an endothermic reaction it would absorb the heat of the surrounding to provide energy for said reaction.
 Source: quizlet.com
Source: quizlet.com
Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Exothermic sweating would make Speed the movie a lot more exciting. Evaporation is an endothermic process and thus requires energy. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Because molecules are on the inside of.
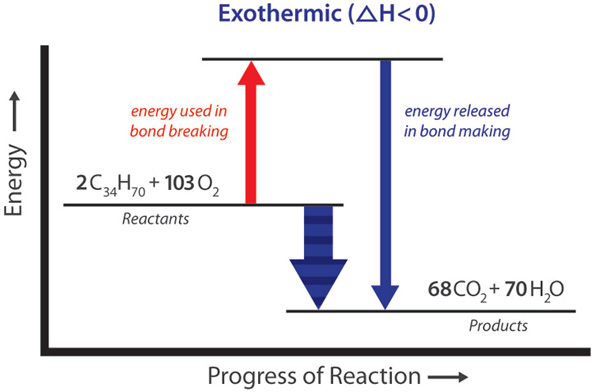 Source: highschoolenergy.acs.org
Source: highschoolenergy.acs.org
Evaporation of alcohol from the skin here the heat is absorbed by the alcohol so that it gets evaporated from the skin and it is an endothermic process. Sweat aqheat-sweat g Endothermic means that you need to put heat energy in to make the reaction happen. In addition to the formation of new compounds the chemical reaction that takes place when ethanol is burnt also releases a lot of heat 2777 kJ per mole of ethanol. Because molecules are on the inside of. Evaporation is an endothermic process.
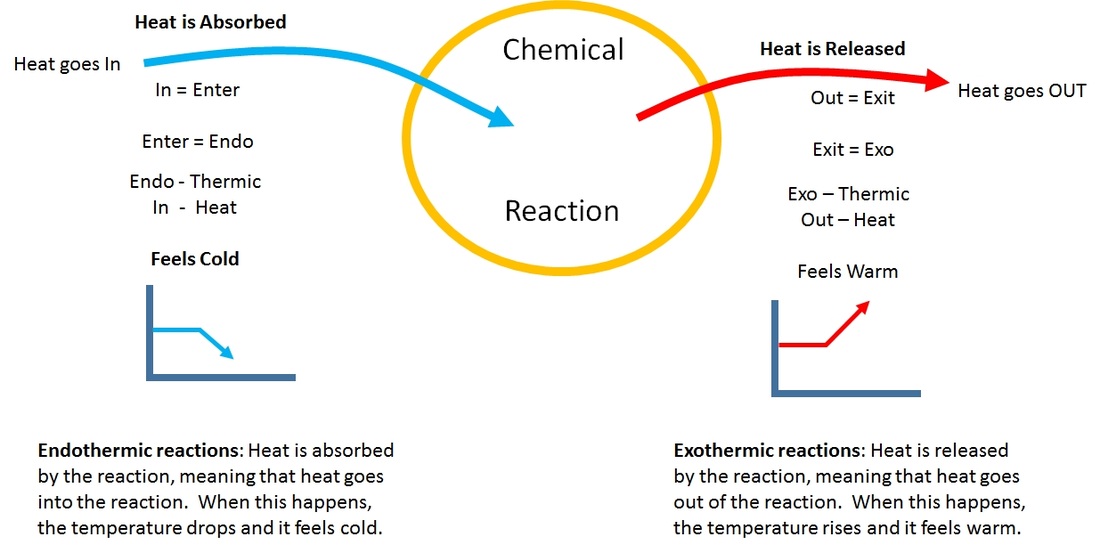 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Endothermic must be supplied with heat and is basically the opposite of exothermic. The particles that make up a liquid sample will have less. Since Energy is Absorbed to break the bonds before it can become a gas that makes Evaporation an Endothermic Process. Is the evaporation of alcohol endothermic or exothermic. Exothermic sweating would make Speed the movie a lot more exciting.
 Source: pinterest.com
Source: pinterest.com
In addition to the formation of new compounds the chemical reaction that takes place when ethanol is burnt also releases a lot of heat 2777 kJ per mole of ethanol. To define endothermic and evaporation is endothermic we need something like this. The process of breaking bonds is endothermic and the process of making bonds is exothermic. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____. Evaporation of alcohol from the skin here the heat is absorbed by the alcohol so that it gets evaporated from the skin and it is an endothermic process.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
I would thus guess that formation of most carboxylic esters from carboxylic acids and alcohols would be slightly exothermic or slightly endothermic depending on structure. 2 A system that can exchange heat with the surroundings. 24 Votes The combustion of ethanol is an exothermic reaction. So for an endothermic reaction it would absorb the heat of the surrounding to provide energy for said reaction. Evaporation is an endothermic process.
 Source: footprints-science.co.uk
Source: footprints-science.co.uk
The result is less heat when the reaction is done. There must be heat added or absorbed from the environment to cook the egg or any other food item. Evaporation is a physical process whereby water in li An Exothermic Reaction will have an Exothermic change whereby energy In the form of heat is releasegiven out to the surrounding. Vaporization represents a change in state from liquid to gas. I would thus guess that formation of most carboxylic esters from carboxylic acids and alcohols would be slightly exothermic or slightly endothermic depending on structure.
 Source: pinterest.com
Source: pinterest.com
So for an endothermic reaction it would absorb the heat of the surrounding to provide energy for said reaction. An endothermic change is a change that takes in heat. DeltaH for an endothermic process is _____ while DeltaH for an exothermic process is _____. Sweat aqheat-sweat g Endothermic means that you need to put heat energy in to make the reaction happen. Remember exo means to exit while thermic means heat Complete the following chart for Water Evaporation or Boiling Point Condensation.
Source: quora.com
Feb 9 2012. If you put rubbing alcohol onto your skin it will evaporate and your skin will feel cold. Condensation would be exothermic. So for an endothermic reaction it would absorb the heat of the surrounding to provide energy for said reaction. Because molecules are on the inside of.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
In fact what happens is that the temperature of reactants and products is reduced without absorbing heat from the surroundings. Positive negative A chemical reaction that absorbs heat from the surroundings is said to be _____ and has a ______ DeltaH at constant pressure. We review their content and use your feedback to keep the quality high. Is a hot cup of coffee system cools on a countertop endothermic or exothermic. Is the freezing of water endothermic or exothermic.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is evaporation of ethanol endothermic or exothermic by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.