Your Is vaporization endothermic or exothermic images are available. Is vaporization endothermic or exothermic are a topic that is being searched for and liked by netizens today. You can Find and Download the Is vaporization endothermic or exothermic files here. Get all royalty-free photos.
If you’re looking for is vaporization endothermic or exothermic images information related to the is vaporization endothermic or exothermic topic, you have come to the right site. Our website frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly surf and find more informative video content and graphics that fit your interests.
Is Vaporization Endothermic Or Exothermic. Yes vaporization is an endothermic reaction while freezing is an exothermic reaction. Evaporation is an endothermic process. The particles that make up a liquid sample will have less. Think of a pot of water set of the stove.
 Ectothermic Endothermic Exothermic Reaction Chemistry How To Get Warm From pinterest.com
Ectothermic Endothermic Exothermic Reaction Chemistry How To Get Warm From pinterest.com
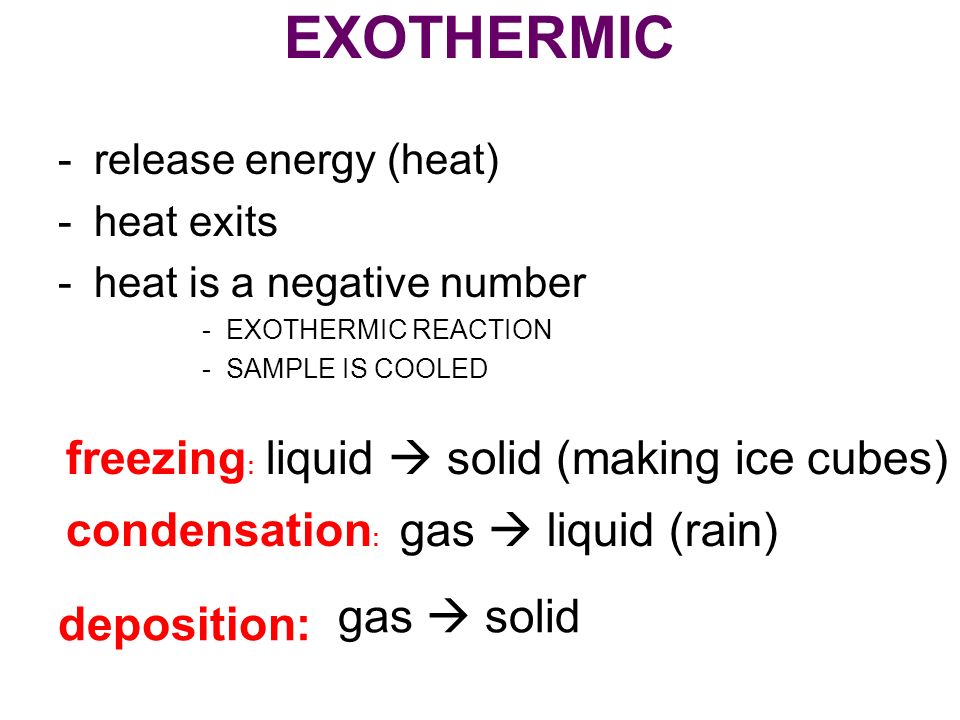
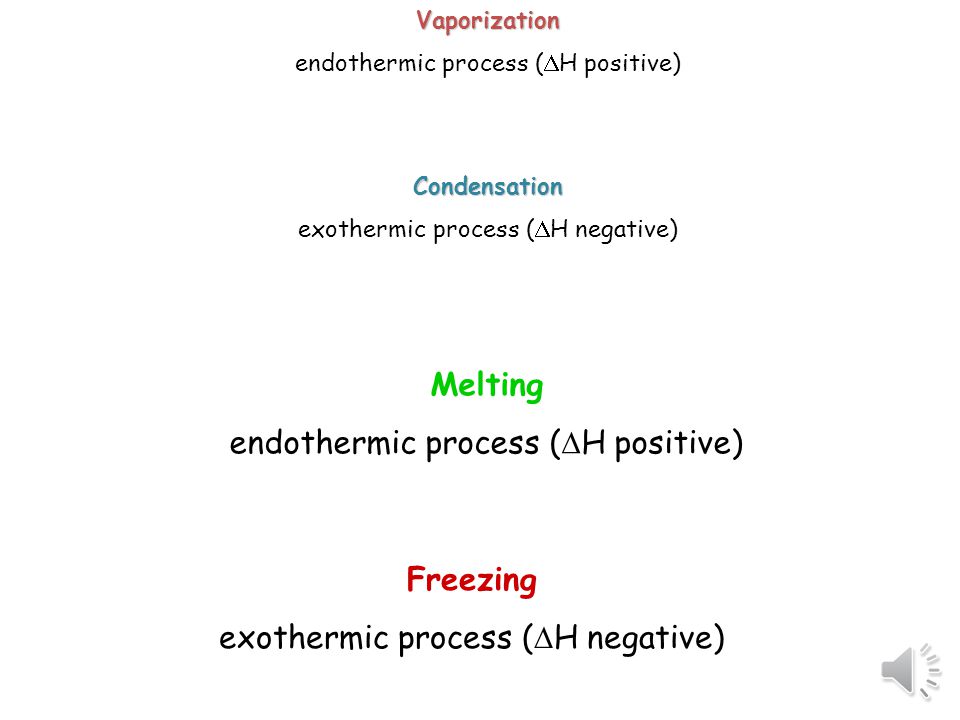
Changes of state are examples of phase changes or phase transitions. The following are the changes of state. So it is an exothermic process and releases the caloric amount of Latent Heat of Vaporization for the mass of steam that condenses. Vaporization like melting requires energy to be put in for the molecules to change state from liquid to gas. Click to see full answer. In chemistry vaporization is defined as the transformation of a solid or liquid into gas.
The particles that make up a liquid sample will have less.
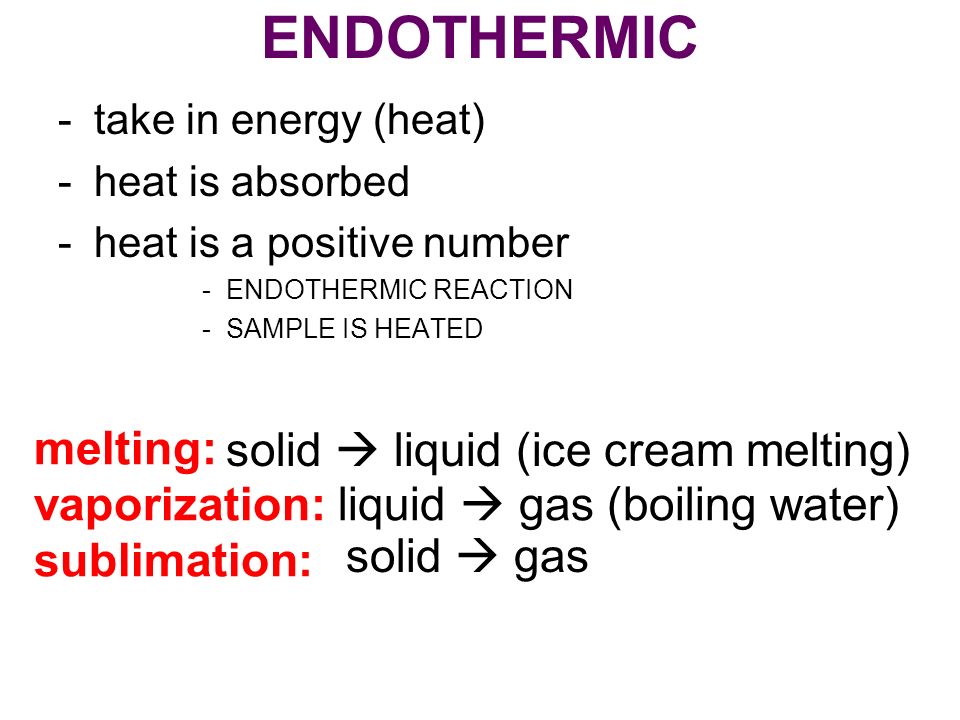
Explain the difference between the two forms of vaporization. This is an exothermic reaction because heat is technically given off in order for the gas to cool and change state. If heat is added to a substance such as in melting vaporization and sublimation the process is endothermic. The following are the changes of state. All phase changes are accompanied by changes in the energy of. The term endothermic process describes a process or reaction in which the system absorbs energy from its.
 Source: slideplayer.com
Source: slideplayer.com
Changes of state are examples of phase changes or phase transitions. Simply so is vaporization endothermic or exothermic. The following are the changes of state. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Explain the difference between the two forms of vaporization.
 Source: pinterest.com
Source: pinterest.com
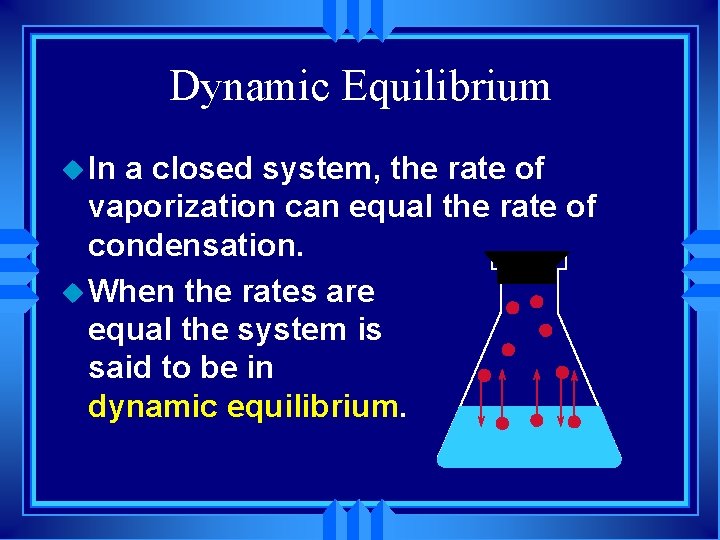
As time passes and water temperature drops the gas molecules water vapor around that glass slow down and change state from gas to liquid as they collect on the surface of the glass. So it is an exothermic process and releases the caloric amount of Latent Heat of Vaporization for the mass of steam that condenses. Think of a pot of water set of the stove. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. The temporary bonds between liquid molecules must be broken for the liquid to change to a gas this requires energy to be put into the system endothermic Exothermic means heat out.
 Source: gr.pinterest.com
Source: gr.pinterest.com
Vaporization like melting requires energy to be put in for the molecules to change state from liquid to gas. Changes of state are examples of phase changes or phase transitions. Yes vaporization is an endothermic reaction while freezing is an exothermic reaction. Explain the difference between the two forms of vaporization. Boiling occurs throughout the liquid while evaporation only occurs at the surface.
 Source: slidetodoc.com
Source: slidetodoc.com
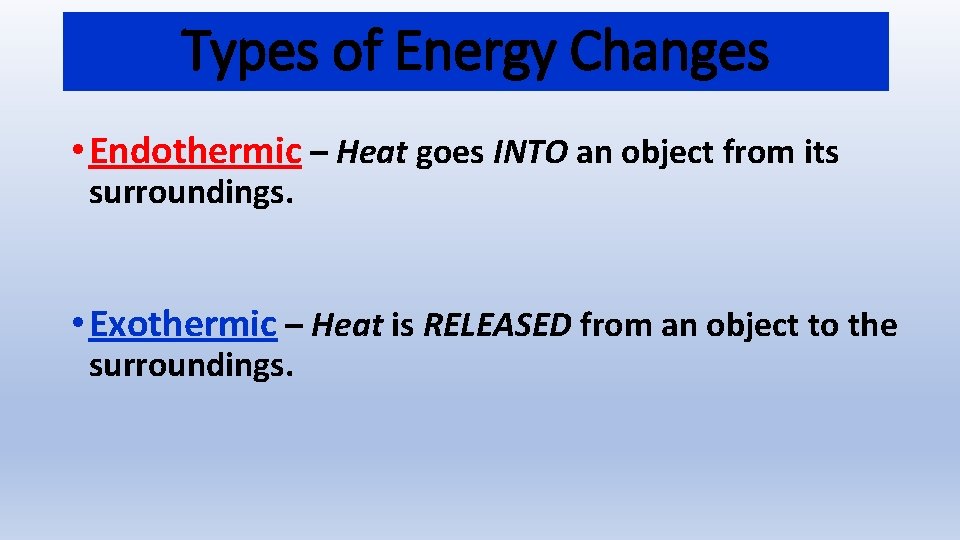
During an endothermic change of state energy is added to the particles while in an exothermic change of state energy is removed. Changes of state are examples of phase changes or phase transitions. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Endothermic and Exothermic Reactions in this video were going to talk about endothermic and exothermic reactions so in an endothermic reaction Delta H the.
 Source: slidetodoc.com
Source: slidetodoc.com
If heat is added to a substance such as in melting vaporization and sublimation the process is endothermic. Evaporation is a type of vaporization of a liquid that occurs from the surface of a liquid into gaseous phase that is not saturated with the evaporating substance. The particles that make up a liquid sample will have less. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Changes of state are examples of phase changes or phase transitions.
 Source: slidetodoc.com
Source: slidetodoc.com
Youre putting inheat in order to break. The temporary bonds between liquid molecules must be broken for the liquid to change to a gas this requires energy to be put into the system endothermic Exothermic means heat out. Compare an endothermic change of state and an exothermic change of state. Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy. Click to see full answer.
 Source: socratic.org
Source: socratic.org
Compare an endothermic change of state and an exothermic change of state. So think of liquid water if you want to vaporize the interesting do you need to add heat or do needs removing vaporization the process of converting a liquid into a gas is always an. What are you doing to it. Explain the difference between the two forms of vaporization. If heat is added to a substance such as in melting vaporization and sublimation the process is endothermic.
 Source: pinterest.com
Source: pinterest.com
Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. The following are the changes of state. Vaporization of liquid alcohol or any liquid is an endothermic process. So think of liquid water if you want to vaporize the interesting do you need to add heat or do needs removing vaporization the process of converting a liquid into a gas is always an. So it is an exothermic process and releases the caloric amount of Latent Heat of Vaporization for the mass of steam that condenses.
 Source: slideplayer.com
Source: slideplayer.com
So it is an exothermic process and releases the caloric amount of Latent Heat of Vaporization for the mass of steam that condenses. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Endothermic reactions end up with an overall positive heat of reaction. One familiar example is sweat which cools the human body as it evaporates from the skin. Endothermic and Exothermic Reactions in this video were going to talk about endothermic and exothermic reactions so in an endothermic reaction Delta H the.
 Source: slideplayer.com
Source: slideplayer.com
Vaporization is an example of an endothermic reaction. Changes of state are examples of phase changes or phase transitions. Endothermic means heat in. All phase changes are accompanied by changes in the energy of a system. Simply so is vaporization endothermic or exothermic.
 Source: pinterest.com
Source: pinterest.com
Click to see full answer. One familiar example is sweat which cools the human body as it evaporates from the skin. These types of reactions occur when the temperature of the system decreases and the surroundings gain energy. Changes of state are examples of phase changes or phase transitions. Changes of state are examples of phase changes or phase transitions.
 Source: brainly.com
Source: brainly.com
The same amount of heat will be released when the steam condenses to liquid water at 100 deg. Exothermic and endothermic reactions both cause some type of energy level differences. Click to see full answer. Explain the difference between the two forms of vaporization. Vaporization of liquid alcohol or any liquid is an endothermic process.
 Source: gr.pinterest.com
Source: gr.pinterest.com
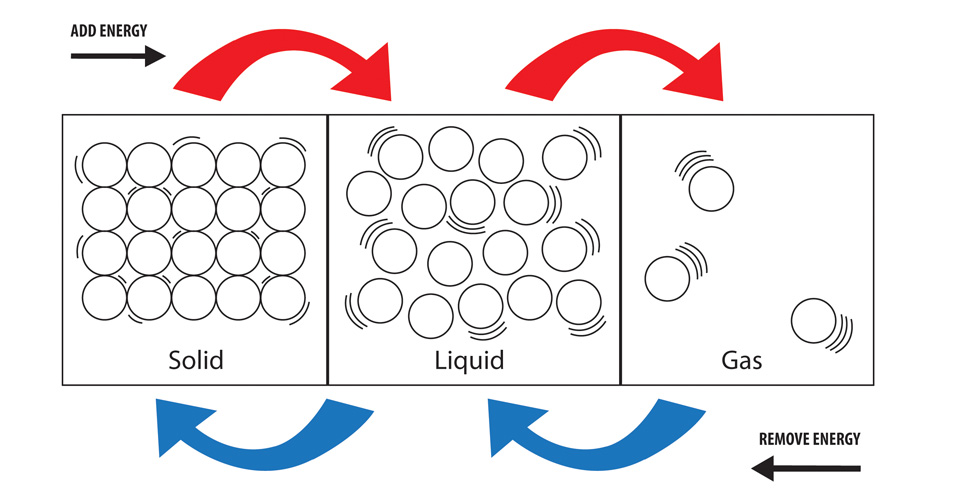
Changes of state are examples of phase changes or phase transitions. In the right-hand container the substance is a solid which takes neither the shape nor the volume of its container. Click to see full answer Similarly you may ask is evaporation of water is an exothermic process. Endothermic reactions absorb energy from the reactions instead of releasing energy into their surroundings. Think of a pot of water set of the stove.
 Source: slideplayer.com
Source: slideplayer.com
This is caused by either evaporation or boiling. What are you doing to it. The term endothermic process describes a process or reaction in which the system absorbs energy from its. Click to see full answer. As time passes and water temperature drops the gas molecules water vapor around that glass slow down and change state from gas to liquid as they collect on the surface of the glass.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
Compare an endothermic change of state and an exothermic change of state. So it is an exothermic process and releases the caloric amount of Latent Heat of Vaporization for the mass of steam that condenses. Click to see full answer Similarly you may ask is evaporation of water is an exothermic process. The same amount of heat will be released when the steam condenses to liquid water at 100 deg. One familiar example is sweat which cools the human body as it evaporates from the skin.
 Source: slideplayer.com
Source: slideplayer.com
What are you doing to it. The term endothermic process describes a process or reaction in which the system absorbs energy from its. Compare an endothermic change of state and an exothermic change of state. Click to see full answer Similarly you may ask is evaporation of water is an exothermic process. As time passes and water temperature drops the gas molecules water vapor around that glass slow down and change state from gas to liquid as they collect on the surface of the glass.
 Source: slideplayer.com
Source: slideplayer.com
Click to see full answer. The term endothermic process describes a process or reaction in which the system absorbs energy from its. In the right-hand container the substance is a solid which takes neither the shape nor the volume of its container. This is an exothermic reaction because heat is technically given off in order for the gas to cool and change state. Vaporization is an example of an endothermic reaction.
 Source: pinterest.com
Source: pinterest.com
Click to see full answer. Endothermic reactions end up with an overall positive heat of reaction. Endothermic and Exothermic Reactions in this video were going to talk about endothermic and exothermic reactions so in an endothermic reaction Delta H the. All phase changes are accompanied by changes in the energy of a system. Think of a pot of water set of the stove.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is vaporization endothermic or exothermic by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





